Rate of Reaction of Magnesium and Hydrochloric Acid
Introduction
In acid-base chemical reactions, there are four main variables, which influence the rate of reaction. These include the presence or absence of catalyst, temperature, concentration, and surface area of reactants. Temperature influences the rates of reaction through kinetic energy, such that high temperatures increase the kinetic energy of reacting molecules therefore causing frequent collisions, which form products faster. High concentrations imply that more reacting molecules are at high proximity to each other therefore intermolecular collisions are frequent therefore forming products frequently. Reactants with high surface area provide a greater binding surface for other reacting molecules, and therefore increase the number of successful collisions at any moment.
To measure, the effect of each of above factors, one has to hold some factors constant during rate reaction experimentation. Therefore, this study intends to investigate the effect of concentration and surface area of reactants on the rate of chemical reactions.Magnesium metal (in form of a ribbon or powder) reacts with acids rapidly than water liberating hydrogen gas. For stance, magnesium metal reacts with hydrochloric to form magnesium chloride salt while displacing hydrogen from the acid as hydrogen gas. This is as shown in the equation below:2HCl (aq) + Mg (s) => MgCl2 (aq) + H2 (g)Research Question: If magnesium ribbon is replaced with an equivalent weight of powered magnesium, does the rate of reaction between magnesium and hydrochloric acid double?
Aims and objectives of the experiment
The aim of this experiment is to verify the effects of surface area of solid reactants and concentration of aqueous reactants on the rates of acid-base chemical reactions. Therefore, we sought to test the duration of reaction of equal lengths Magnesium ribbons with reducing concentrations of hydrochloric acid.
Similarly, the duration of reaction will be determined using equivalent weights of powdered Magnesium metal. The experiment will be carried at a room temperature 25 0C.
Study Variables
- The Dependent Variables: The measured duration of acid-metal reaction in seconds and the rate of gas bubbles.
- The Independent Variables: The concentration of hydrochloric acid used and the surface area of the Magnesium metal used.
- The Controlled Variables (constants): The quantity of the Magnesium for each test will be held constant.
For the Magnesium ribbon, the lengths of the Magnesium ribbon used will be constant, while quantities of powdered Magnesium metal (in grams) will be equivalent to the weight of the length of magnesium ribbon used. The experiment will be carried out at room temperature (25 0C)
The study variables are summarized in the table below:
| Variables | Operationalization of variables |
| The dependent variables | The duration of reaction, (time taken for Magnesium to dissolve in hydrochloric acid completely) measured using a stopwatch in seconds.The rate of gas bubbles |
| The independent variables | Increasing concentration of hydrochloric acid: This will be changed by changing dilution factor.Surface area of Magnesium ribbon: This will be changed by using Magnesium ribbons and powdered Magnesium metal in separate experiments. |
| The constants (controlled variables) | The quantity of Magnesium metal used will be held constant by way of using equal lengths of Magnesium ribbons and equivalent weights (in grams) of powdered Magnesium metal.All the reaction will be carried out under a constant temperature (room temperature of 25 0C).
|
Table 1 A table of study variables and operationalization of the study variablesPredictionGiven that, powdered Magnesium metal has a high surface area than equivalent lengths of Magnesium ribbon, we predict that the former will have shorter duration of reaction with hydrochloric acid than the latter. We also predict that reaction of powdered Magnesium metal with highest concentration of hydrochloric acid will take the shortest duration of reaction.Hypothesis: Powdered Magnesium metal will reduce the reaction duration by a half if used in place of equivalent length of magnesium ribbon, when reacted with hydrochloric acid.
Equipment and Materials
Chemicals and ReagentsThe following chemicals and reagents were required in the experimentation:
- Magnesium ribbon
- Powdered Magnesium metal
- Standardized hydrochloric acid concentrations (3.0 M, 2.
0 M, 1.5M, 1.0 M and 0.5M )
- Distilled water
Apparatus and personal protection equipment
- 10 Conical flasks (100 cm3)
- 3 Measuring cylinders (100 cm3)
- Clamp stand
- Glass trough
- A Stopwatch
- Safety goggles
- Laboratory dust coat
Experimentation procedures
The experiment procedure was divided into two related investigations involving equal lengths of Magnesium ribbons and equal amounts of powdered Magnesium metal.
- First, repair your working bench by simply removing unnecessary materials. Make sure you put on your personal protective clothing and safety goggles.
- Clean the Magnesium ribbon using a sand paper to remove oxides coating its surface. This will reduce reaction errors related to impurities.
- Cut 5 equal sizes (10 cm) pieces of Magnesium from the fleshly cleaned Magnesium ribbon, weigh each of them using a digital weighing balance and record their weights.
- Wrap the magnesium pieces immediately in an aluminum foil to prevent them from being re-oxidized.
- Measure 40 ml of 3M HCl using a clean dry measuring cylinder and pour into a clean 100 ml conical flask.
- Add 40 ml of distilled water and label the conical flask with the concentration of the HCL poured.
- Repeat step 5 and 6 for 2M, 1.5M, 1M, and 0.5M HCL and keep all the acids ready on the working bench.
- Reset your stopwatch timer and prepare a gas delivery system including water bath as shown figure one below.
- Pick one piece of Magnesium ribbon drop in the first prepared acid in the conical flask and immediately start your stopwatch.
- Immediately cork the flask to the prepared gas delivery system.
- Monitor the reaction progress closely and stop your running stopwatch when the Magnesium ribbon completely dissolves in the acid and record the reaction duration in seconds in a data sheet.
- Reset your stopwatch, a repeat steps 9, 10 and 11 for the subsequent acids.
- Discard all the chemicals, wash, and rinse the conical flasks ready for another procedure.
- Repeat steps 5, 6 and 7 above.
- Add up the weights of the five 10 cm-long magnesium ribbons and obtain the average weight in grams
- Use the average weight as obtained in 15 above and weigh of an equivalent weight of Magnesium powder (for this case 0.102 grams) and pour into the first conical flask containing the 3 M HCl acid, start your stopwatch, and immediately cork the flask to the gas delivery system.
- Monitors the reaction progress and stop the stopwatch when the Magnesium powder dissolves completely in the acid.
- Repeat step 16 with 2M, 1.5M, 1M, and 0.
5M HCL and clearly label your results.
- Clear your working bench.
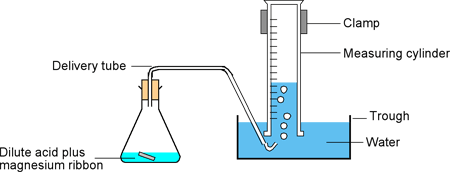 Figure 1: Experimental set-up of HCl-Magnesium reaction
Figure 1: Experimental set-up of HCl-Magnesium reaction
Results and Observations
During the reaction, the water bath in the gas delivery system showed gas bubbles ascending to the gas cylinder.
At higher acid concentration, the rates of bubble forming were rapid than those in lower acid concentrations were. The most rapid gas bubbles were observed in the acid reactions with powdered Magnesium metal. The duration of reactions were recorded as shown in tables 2 and 3 below.
| Concentration of HCl (M) | 3.0 | 2.0 | 1.
5 |
1.0 | 0.5 |
| Reaction duration (sec) | 37 | 51 | 77 | 158 | 201 |
Table 2. A table of results showing HCl-Magnesium ribbon reaction duration (seconds) in reducing concentration
| Concentration of HCl (M) | 3.0 | 2.0 | 1.
5 |
1.0 | 0.5 |
| Reaction duration (sec) | 16 | 24 | 48 | 89 | 117 |
Table 3. A table of results showing HCl-Magnesium powder reaction duration (seconds) in reducing concentration
Processing and Presenting Data
Graph
Importantly, suitable acid-base indicators can be used to detect the end of the reaction accurately.
References
- Barrans, R. E. (2012, March). Surface Area and Rate of Reaction.
Retrieved March 8, 2012, from newton.dep.anl.gov: http://www.newton.dep.
anl.gov/askasci/chem00/
- chem00021.htm
- Clark, J. (2002). THE EFFECT OF SURFACE AREA ON REACTION RATES.
Retrieved March 8, 2012, from chemguide.co.uk: http://www.chemguide.co.uk/physical/basicrates/surfacearea.
html
- Gallagher, R., & Ingram, P. (2001). Chemistry for higher tier: New coordinated science. New York: Oxford University Press. p137

